What is Hydrogen Water?
Hydrogen water, also referred to as hydrogen-rich or hydrogenated water, is ordinary water infused with molecular hydrogen gas (H₂). This process involves dissolving hydrogen gas into water under high pressure, creating a supersaturated solution. The hydrogen molecules are extremely small, so they can easily penetrate water and stay dissolved for a while.
In recent years, hydrogen-rich water has attracted considerable interest as a potential health-boosting beverage. Research conducted on both animals and humans over the past few decades has suggested that hydrogen-infused water may exhibit antioxidant, anti-inflammatory, and cell-protective properties.
A common question we get is: “Since water is H2O, there is already hydrogen in water, so why can’t we just drink water to get hydrogen?”
First, let’s start with the water molecule H2O.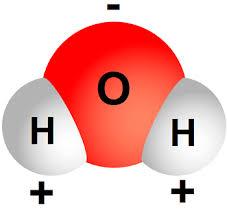
The water molecule is composed of two hydrogen atoms, each linked by a chemical bond to an oxygen atom. Just as you can’t drink water to get the oxygen you need, the “hydrogen” in the water does not “come off” when you drink it. It stays as a part of the water molecule, which is a very stable molecule. Unless something is done to break the chemical bond.
So what then is hydrogen water?
Hydrogen water (or sometimes called hydrogen-infused water or hydrogen-rich water) is water that contains hydrogen molecules. These hydrogen molecules exist in the water in gaseous form. Simply put, hydrogen water is water with hydrogen gas suspended in it.
How do you extract hydrogen from water?
The most efficient method of producing hydrogen molecules from water is by splitting the water molecule H2O into its components, hydrogen and oxygen, through a process called electrolysis.
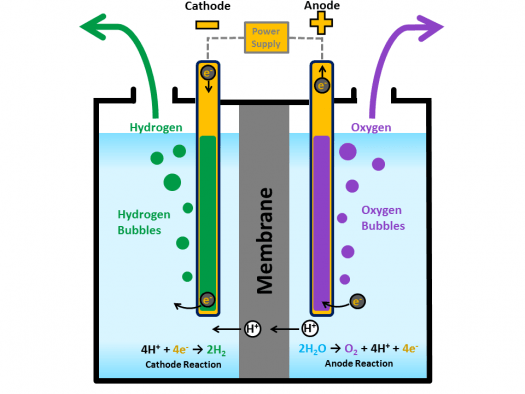
During electrolysis an electric current is passed through the water. This produces a chemical reaction in which water is broken down into oxygen and hydrogen: 2 H2O → 2 H2 + O2
Water splitting into hydrogen and oxygen
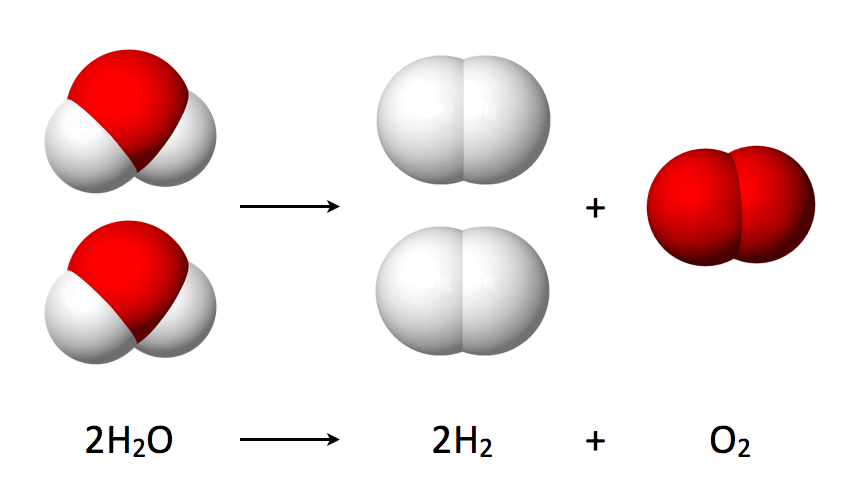
Once separated, the hydrogen molecules (H2) become a gas. They are then mixed with water to form a solution that is rich in hydrogen gas. Hence, when you drink hydrogen water you are in fact drinking water + hydrogen gas.